Title: A Frame Shift Mutation in the Fibrinogen A Alpha Chain Gene in a Kindred With Renal Amyloidosis (1)
Authors: Tomoyuki Uemichi, Juris J. Liepnieks, Toshiyuki Yamada, Morie A. Gertz, Nils Bang, and Merrill D. Benson (Indiana University School of Medicine, Indianapolis, IN, USA and Mayo Clinic, Rochester, MN, USA)
Journal: Blood (1996)
Abstract:
A new American kindred with amyloidosis was found by single-strand conformation polymorphism analysis to have a mutation in the fibrinogen A alpha chain gene. Affected members in this kindred have autosomal dominant amyloid nephropathy. DNA sequencing showed a single nucleotide deletion at the third base of codon 524 of the fibrinogen A alpha chain genes (4904delG) that resulted in a frame shift and premature termination of the protein at codon 548. Antiserum was produced to a portion of the abnormal peptide predicted by the DNA sequence and amyloid deposits were immuno-histologically proven to contain this abnormal peptide. Two of the propositus' 4 children were positive for the mutant fibrinogen A alpha chain gene by restriction fragment length polymorphism analysis based on polymerase chain reaction. These two mutant gene carriers now in the second decade of life show no clinical symptoms of amyloidosis as yet but have lower plasma fibrinogen concentrations when compared with their normal siblings. This [is] the first description of a kindred with renal amyloidosis and low plasma fibrinogen and also the first report of amyloidosis caused by a frame shift mutation.Here is a link to the PDF of the article, if you'd like to follow along: http://bloodjournal.hematologylibrary.org/content/87/10/4197.full.pdf
As mentioned in the abstract, this article reports on a US kindred found to have a fibrinogen mutation. The propositus (first family member diagnosed) was a woman who was diagnosed with proteinuria at the age of 41 and died after developing cardiac failure and renal insufficiency at the age of 46. A kidney biopsy at the age of 42 showed amyloid deposits in the glomeruli. An autopsy showed amyloid deposits in both kidneys but not in the heart.
Her mother died at the age of 38 due to renal amyloidosis, and one of her mother's brothers died of renal failure at the age of 41.
We'll skip the Materials and Methods section since it's just the details of the laboratory processes of isolating fibrinogen from the affected patient, autopsied tissues, and possibly affected relatives.
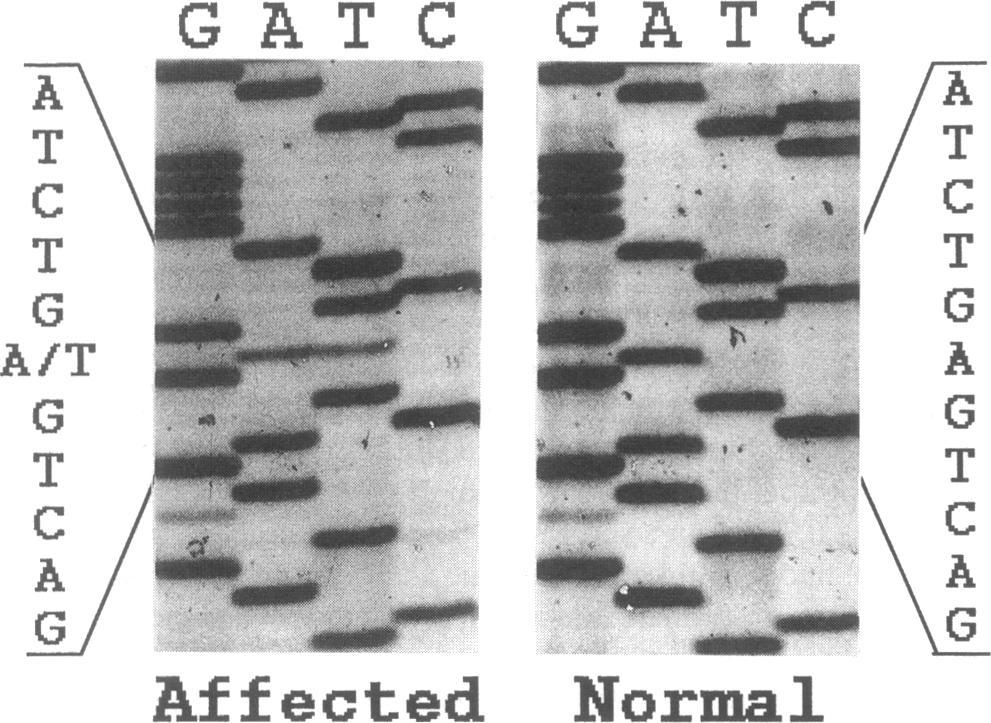
The Results section of the article describes the actual mutation that was discovered. This mutation was on the fibrinogen A alpha chain, but instead of a substitution like the previous two mutations (where a G, A, T or C at one position in the DNA is substituted for one of the other three letters), this mutation was a deletion at one position (4904), which shifted everything else beyond that position by one. (That's why they use the term "frame shift mutation.") Since these letters are in groups of three to determine each amino acid in the sequence, each amino acid after this point was also wrong because of this shift. But not only that, this shift also caused the string to end prematurely 25 amino acids later because a particular three-letter sequence came up that signals the end of the string. I'll give a musical analogy to help you picture what this frame shift mutation really means.
Think of the four main building blocks of DNA (represented by the letters G, A, T and C) as musical notes. These four building blocks assemble in groups of three to form amino acids, so think of amino acids as three-note musical chords. In the case of a substitution mutation such as Glu526Val, when these chords are played in order everything sounds fine up to the point of the mutation, then there is one wrong chord, then everything sounds fine after that. But in the case of a frame shift mutation like the one in this article, all the chords sound fine until the mutation is reached, and then every chord after that is likely to be wrong because it will have two notes from the correct chord and one note from the next chord. Plus, in this particular case, the chords stop prematurely.
Next they describe the other interesting difference between this mutation and the previously described mutations. You may recall that the 1994 article showed the Glu526Val mutation had no impact on blood clotting times. The mutation described in this article from 1996, however, does seem to have an impact on blood clotting times and plasma fibrinogen concentration levels. They found the blood clotting times were prolonged, and plasma fibrinogen concentration levels were at or below normal levels. These changes were not enough to be classified as hypofibrinogenemia (a deficiency of fibrinogen in the blood), but there is some measurable impact. So if you have this particular mutation you should inform your doctor that there is an impact on blood clotting times. (I suppose that should be considered when prescribing anticoagulants, for instance.)
The Discussion section of the article begins with a general overview of the structure of fibrinogen and various mutations known to cause blood clotting disorders. It then states there are three kindreds in previously published reports that have fibrinogen A alpha chain mutations known to cause hereditary renal amyloidosis. So the kindred in this report is only the fourth one to be reported with such a mutation. (Three mutations, four families.)
Although they could not test any tissue samples from the propositus' mother, it is likely that she had the mutation because she died of renal amyloidosis at the age of 38, and the propositus' father does not have the mutation. (The Results section also mentioned that two of the propositus' four children tested positive for the mutation but do not have any symptoms.)
The age of onset seems to be different for the three mutations that have been reported up to this point. The earliest age of onset occurs with the first reported mutation (Arg554Leu). Those patients were affected in their 20s or 30s. For the frameshift mutation in this article, the age of onset appears to be in the late 30s or early 40s. Finally, for the Glu526Val mutation, the age of onset has been in the 40s through 60s.
This section then has a paragraph about treatment. Hemodialysis is mentioned since that is a standard treatment for other renal diseases. Regarding kidney transplant, one patient with the Arg554Leu mutation did receive a kidney transplant which functioned for 10 years before failing due to recurrence of amyloid in the transplanted kidney. It then mentions liver transplantation, which has promising results so far in treating patients with the most common type of familial amyloidosis, related to transthyretin mutations. The paragraph closes with this sentence, which definitely becomes important in the future as treatment options evolve: "Because fibrinogen is also largely synthesized in the liver, amyloidosis patients with variant fibrinogen may also be candidates for liver transplantation."
The article concludes with some discussion on the possible reasons why the three mutations are common in terms of the impact on the kidneys, yet this mutation is the only one of the three that affects clotting times. One likely reason is since this mutation is at amino acid location 524 out of 610, the final 14% of the sequence is wrong or missing.
=====
So as of 1996, three fibrinogen mutations have been reported that cause amyloidosis. They are similar in terms of progressing to kidney failure, but there are some differences with regard to age of onset and affect on blood clotting. Dialysis and kidney transplantation are the only treatment options used so far, but liver transplantation is being proposed as a viable treatment option as well.
=====
Citation
(1) Uemichi T, Liepnieks JJ, Yamada T, Gertz MA, Bang N, Benson MD. A frame shift mutation in the fibrinogen A alpha chain gene in a kindred with renal amyloidosis. Blood 1996; 87:4197-203.